How Nutrients Are Moved From Available to Unavailable Soil Pools and Then Back Again.
Abstract
Nitrogen, the most abundant element in our temper, is crucial to life. Nitrogen is found in soils and plants, in the water nosotros drink, and in the air we exhale. It is also essential to life: a key building block of DNA, which determines our genetics, is essential to plant growth, and therefore necessary for the nutrient nosotros abound. But as with everything, balance is cardinal: as well little nitrogen and plants cannot thrive, leading to low crop yields; but too much nitrogen can be toxic to plants, and can also harm our surroundings. Plants that do not accept enough nitrogen become yellowish and do not grow well and can have smaller flowers and fruits. Farmers can add nitrogen fertilizer to produce better crops, just too much can hurt plants and animals, and pollute our aquatic systems. Agreement the Nitrogen Bicycle—how nitrogen moves from the atmosphere to earth, through soils and back to the atmosphere in an endless Bicycle—can help the states abound healthy crops and protect our surroundings.
Introduction
Nitrogen, or N, using its scientific abbreviation, is a colorless, odorless element. Nitrogen is in the soil nether our anxiety, in the water we drink, and in the air we breathe. In fact, nitrogen is the most abundant element in Globe'south atmosphere: approximately 78% of the atmosphere is nitrogen! Nitrogen is important to all living things, including u.s.a.. It plays a fundamental role in establish growth: as well little nitrogen and plants cannot thrive, leading to low ingather yields; but too much nitrogen can exist toxic to plants [1]. Nitrogen is necessary for our food supply, but excess nitrogen tin harm the environment.
Why Is Nitrogen Important?
The delicate balance of substances that is of import for maintaining life is an important surface area of research, and the residual of nitrogen in the environment is no exception [2]. When plants lack nitrogen, they go yellowed, with stunted growth, and produce smaller fruits and flowers. Farmers may add fertilizers containing nitrogen to their crops, to increment crop growth. Without nitrogen fertilizers, scientists approximate that we would lose up to one third of the crops we rely on for food and other types of agronomics. But nosotros need to know how much nitrogen is necessary for plant growth, because too much can pollute waterways, hurting aquatic life.
Nitrogen Is Cardinal to Life!
Nitrogen is a key element in the nucleic acids DNA and RNA , which are the well-nigh important of all biological molecules and crucial for all living things. DNA carries the genetic information, which means the instructions for how to make upwards a life form. When plants practice not get enough nitrogen, they are unable to produce amino acids (substances that contain nitrogen and hydrogen and make up many of living cells, muscles and tissue). Without amino acids, plants cannot make the special proteins that the establish cells demand to abound. Without enough nitrogen, found growth is affected negatively. With too much nitrogen, plants produce excess biomass, or organic matter, such as stalks and leaves, but not enough root structure. In extreme cases, plants with very high levels of nitrogen absorbed from soils can poison farm animals that eat them [3].
What Is Eutrophication and can It Be Prevented?
Excess nitrogen can as well leach—or drain—from the soil into underground water sources, or it tin can enter aquatic systems every bit above basis runoff. This excess nitrogen can build up, leading to a procedure called eutrophication . Eutrophication happens when too much nitrogen enriches the h2o, causing excessive growth of plants and algae. Also much nitrogen can even cause a lake to turn bright green or other colors, with a "bloom" of smelly algae called phytoplankton (encounter Figure i)! When the phytoplankton dies, microbes in the water decompose them. The process of decomposition reduces the amount of dissolved oxygen in the water, and tin can pb to a "dead zone" that does not have plenty oxygen to support nearly life forms. Organisms in the dead zone dice from lack of oxygen. These dead zones can happen in freshwater lakes and as well in coastal environments where rivers full of nutrients from agricultural runoff (fertilizer overflow) flow into oceans [4].

- Figure ane - Eutrophication at a waste matter water outlet in the Potomac River, Washington, D.C.
- The water in this river, is bright greenish because information technology has undergone eutrophication, due to backlog nitrogen and other nutrients polluting the water, which has led to increased phytoplankton and algal blooms, so the water has become cloudy and tin turn different colors, such as greenish, yellowish, red, or brown, depending on the algal blooms (Wikimedia Commons: https://commons.wikimedia.org/wiki/Category:Eutrophication#/media/File:Potomac_green_water.JPG).
Figure two shows the stages of Eutrophication (open admission Wikimedia Commons image from https://eatables.m.wikimedia.org/wiki/File:Eutrophicationmodel.svg).
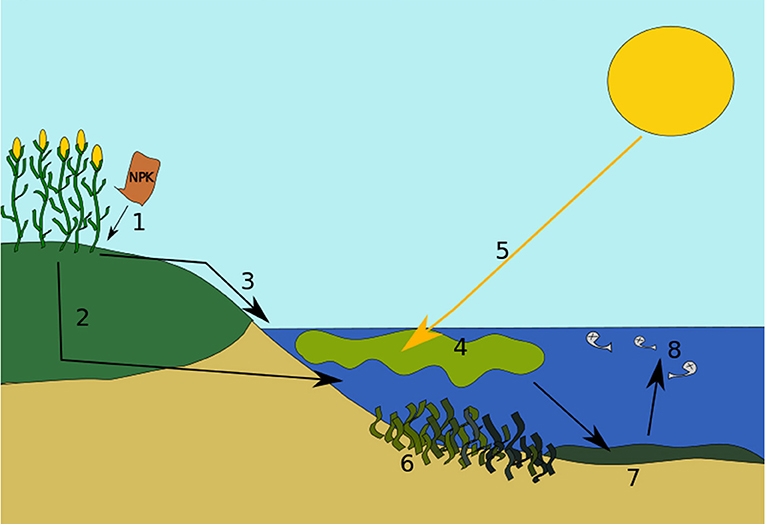
- Figure two - Stages of eutrophication.
- (1) Excess nutrients end upwards in the soil and ground. (two) Some nutrients get dissolved in h2o and leach or leak into deeper soil layers. Eventually, they get drained into a water body, such as a lake or pond. (three) Some nutrients run off from over the soils and ground directly into the water. (four) The extra nutrients cause algae to bloom. (5) Sunlight becomes blocked past the algae. (half dozen) Photosynthesis and growth of plants under the water volition be weakened or potentially stopped. (7) Next, the algae flower dies and falls to the bottom of the water torso. And then, leaner begin to decompose or break up the remains, and apply upward oxygen in the process. (8) The decomposition procedure causes the water to have reduced oxygen, leading to "dead zones." Bigger life forms similar fish cannot breathe and dice. The water torso has now undergone eutrophication.
Can eutrophication be prevented? Aye! People who manage water resources can utilize different strategies to reduce the harmful effects of algal blooms and eutrophication of water surfaces. They can re-reroute excess nutrients away from lakes and vulnerable costal zones, apply herbicides (chemicals used to kill unwanted plant growth) or algaecides (chemicals used to kill algae) to stop the algal blooms, and reduce the quantities or combinations of nutrients used in agricultural fertilizers, amidst other techniques [5]. But, it tin oft be hard to notice the origin of the excess nitrogen and other nutrients.
Once a lake has undergone eutrophication, it is even harder to do damage command. Algaecides can exist expensive, and they also do not correct the source of the problem: the excess nitrogen or other nutrients that acquired the algae bloom in the start place! Another potential solution is chosen bioremediation , which is the process of purposefully changing the nutrient web in an aquatic ecosystem to reduce or control the amount of phytoplankton. For case, h2o managers can introduce organisms that eat phytoplankton, and these organisms can help reduce the amounts of phytoplankton, by eating them!
What Exactly Is the Nitrogen Bike?
The nitrogen cycle is a repeating wheel of processes during which nitrogen moves through both living and non-living things: the atmosphere, soil, h2o, plants, animals and bacteria . In order to move through the unlike parts of the wheel, nitrogen must change forms. In the atmosphere, nitrogen exists as a gas (N2), but in the soils it exists as nitrogen oxide, NO, and nitrogen dioxide, NO2, and when used as a fertilizer, can exist found in other forms, such as ammonia, NH3, which can be processed fifty-fifty farther into a different fertilizer, ammonium nitrate, or NH4NO3.
There are 5 stages in the nitrogen cycle, and we volition now discuss each of them in turn: fixation or volatilization, mineralization, nitrification, immobilization, and denitrification. In this paradigm, microbes in the soil turn nitrogen gas (Northward2) into what is chosen volatile ammonia (NHiii), and then the fixation process is called volatilization. Leaching is where certain forms of nitrogen (such as nitrate, or NO3) becomes dissolved in water and leaks out of the soil, potentially polluting waterways.
Stage 1: Nitrogen Fixation
In this stage, nitrogen moves from the atmosphere into the soil. Earth'due south temper contains a huge pool of nitrogen gas (N2). But this nitrogen is "unavailable" to plants, because the gaseous course cannot be used directly by plants without undergoing a transformation. To exist used by plants, the Northwardtwo must be transformed through a process called nitrogen fixation. Fixation converts nitrogen in the temper into forms that plants can blot through their root systems.
A small corporeality of nitrogen can exist fixed when lightning provides the energy needed for Northward2 to react with oxygen, producing nitrogen oxide, NO, and nitrogen dioxide, NOii. These forms of nitrogen then enter soils through rain or snow. Nitrogen can as well exist fixed through the industrial process that creates fertilizer. This grade of fixing occurs nether high estrus and force per unit area, during which atmospheric nitrogen and hydrogen are combined to form ammonia (NHthree), which may then be candy further, to produce ammonium nitrate (NHivNOiii), a form of nitrogen that can be added to soils and used by plants.
About nitrogen fixation occurs naturally, in the soil, by bacteria. In Figure 3 (higher up), yous can run across nitrogen fixation and exchange of form occurring in the soil. Some leaner adhere to plant roots and have a symbiotic (beneficial for both the constitute and the bacteria) relationship with the plant [6]. The leaner get energy through photosynthesis and, in return, they gear up nitrogen into a form the found needs. The fixed nitrogen is then carried to other parts of the institute and is used to form plant tissues, then the plant can abound. Other leaner live freely in soils or water and can fix nitrogen without this symbiotic human relationship. These bacteria can also create forms of nitrogen that tin be used by organisms.
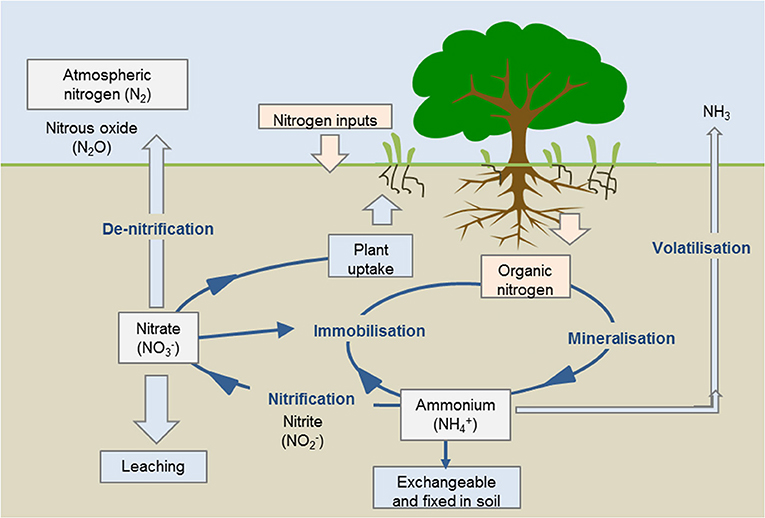
- Effigy iii - Stages of the nitrogen bicycle.
- The Nitrogen Cycle: Nitrogen cycling through the diverse forms in soil determines the amount of nitrogen available for plants to uptake. Source: https://www.agric.wa.gov.au/soil-carbon/immobilisation-soil-nitrogen-heavy-stubble-loads.
Stage 2: Mineralization
This stage takes place in the soil. Nitrogen moves from organic materials, such equally manure or plant materials to an inorganic class of nitrogen that plants tin can utilise. Eventually, the plant's nutrients are used upward and the constitute dies and decomposes. This becomes of import in the second stage of the nitrogen wheel. Mineralization happens when microbes act on organic material, such every bit animal manure or decomposing institute or animal cloth and begin to catechumen it to a form of nitrogen that can exist used by plants. All plants under cultivation, except legumes (plants with seed pods that split in one-half, such as lentils, beans, peas or peanuts) get the nitrogen they require through the soil. Legumes get nitrogen through fixation that occurs in their root nodules, as described in a higher place.
The outset form of nitrogen produced past the process of mineralization is ammonia, NHiii. The NH3 in the soil so reacts with h2o to course ammonium, NH4. This ammonium is held in the soils and is available for use past plants that practise not get nitrogen through the symbiotic nitrogen fixing relationship described higher up.
Stage 3: Nitrification
The tertiary stage, nitrification, also occurs in soils. During nitrification the ammonia in the soils, produced during mineralization, is converted into compounds chosen nitrites, NOii −, and nitrates, NOthree −. Nitrates can be used by plants and animals that consume the plants. Some bacteria in the soil tin turn ammonia into nitrites. Although nitrite is non usable past plants and animals directly, other bacteria can change nitrites into nitrates—a form that is usable past plants and animals. This reaction provides energy for the bacteria engaged in this procedure. The bacteria that nosotros are talking about are called nitrosomonas and nitrobacter. Nitrobacter turns nitrites into nitrates; nitrosomonas transform ammonia to nitrites. Both kinds of bacteria tin human activity but in the presence of oxygen, O2 [7]. The process of nitrification is of import to plants, equally information technology produces an extra stash of bachelor nitrogen that can be absorbed by the plants through their root systems.
Stage 4: Immobilization
The quaternary stage of the nitrogen cycle is immobilization, sometimes described as the opposite of mineralization. These ii processes together control the amount of nitrogen in soils. But like plants, microorganisms living in the soil require nitrogen every bit an free energy source. These soil microorganisms pull nitrogen from the soil when the residues of decomposing plants do not comprise enough nitrogen. When microorganisms take in ammonium (NH4 +) and nitrate (NOiii −), these forms of nitrogen are no longer bachelor to the plants and may crusade nitrogen deficiency, or a lack of nitrogen. Immobilization, therefore, ties up nitrogen in microorganisms. However, immobilization is important because it helps control and remainder the amount of nitrogen in the soils by tying information technology up, or immobilizing the nitrogen, in microorganisms.
Stage v: Denitrification
In the fifth phase of the nitrogen cycle, nitrogen returns to the air every bit nitrates are converted to atmospheric nitrogen (Northward2) by bacteria through the process nosotros call denitrification. This results in an overall loss of nitrogen from soils, as the gaseous form of nitrogen moves into the temper, dorsum where we began our story.
Nitrogen Is Crucial for Life
The cycling of nitrogen through the ecosystem is crucial for maintaining productive and healthy ecosystems with neither also much nor as well little nitrogen. Plant product and biomass (living material) are limited past the availability of nitrogen. Understanding how the found-soil nitrogen bike works tin assistance u.s.a. make meliorate decisions almost what crops to grow and where to grow them, and then we have an adequate supply of nutrient. Noesis of the nitrogen cycle can also help us reduce pollution caused by adding too much fertilizer to soils. Sure plants can uptake more nitrogen or other nutrients, such as phosphorous, some other fertilizer, and tin even be used as a "buffer," or filter, to prevent excessive fertilizer from entering waterways. For example, a study done by Haycock and Pinay [viii] showed that poplar trees (Populus italica) used every bit a buffer held on to 99% of the nitrate entering the underground water flow during winter, while a riverbank zone covered with a specific grass (Lolium perenne Fifty.) held up to 84% of the nitrate, preventing it from entering the river.
As you take seen, non enough nitrogen in the soils leaves plants hungry, while too much of a good affair can exist bad: excess nitrogen tin can poisonous substance plants and even livestock! Pollution of our water sources by surplus nitrogen and other nutrients is a huge problem, as marine life is being suffocated from decomposition of dead algae blooms. Farmers and communities need to work to improve the uptake of added nutrients by crops and treat animal manure waste product properly. We likewise need to protect the natural plant buffer zones that can take upwards nitrogen runoff before it reaches water bodies. But, our current patterns of immigration trees to build roads and other construction worsen this problem, because there are fewer plants left to uptake excess nutrients. Nosotros need to do further research to determine which institute species are best to abound in littoral areas to take upward excess nitrogen. Nosotros also need to observe other ways to fix or avert the problem of excess nitrogen spilling over into aquatic ecosystems. Past working toward a more complete understanding of the nitrogen cycle and other cycles at play in Globe'southward interconnected natural systems, we can better understand how to ameliorate protect World'south precious natural resources.
Glossary
DNA: ↑ Deoxyribonucleic acid, a self-replicating textile which is nowadays in nearly all living organisms as the principal component of chromosomes, and carrier of genetic information.
RNA: ↑ Ribonucleic acid, a nucleic acid present in all living cells, acts every bit a messenger carrying instructions from DNA.
Eutrophication: ↑ Excessive amount of nutrients (such as nitrogen) in a lake or other body of h2o, which causes a dense growth of aquatic plant life, such equally algae.
Phytoplankton: ↑ Tiny, microscopic marine algae (likewise known as microalgae) that require sunlight in order to grow.
Bioremediation: ↑ Using other microorganisms or tiny living creatures to eat and break down pollution in order to clean a polluted site.
Bacteria: ↑ Microscopic living organisms that ordinarily contain only one jail cell and are found everywhere. Bacteria can cause decomposition or breaking down, of organic material in soils.
Leaching: ↑ When a mineral or chemic (such as nitrate, or NOthree) drains away from soil or other ground material and leaks into surrounding expanse.
Legumes: ↑ A member of the pea family: beans, lentils, soybeans, peanuts and peas, are plants with seed pods that split up in half.
Microorganism: ↑ An organism, or living thing, that is too tiny to exist seen without a microscope, such as a bacterium.
Disharmonize of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential disharmonize of interest.
References
[i] ↑ Britto, D. T., and Kronzuker, H. J. 2002. NHiv + toxicity in college plants: a critical review. J. Plant Physiol. 159:567–84. doi: x.1078/0176-1617-0774
[two] ↑ Weathers, K. C., Groffman, P. G., Dolah, Due east. V., Bernhardt, E., Grimm, N. B., McMahon, 1000., et al. 2016. Frontiers in ecosystem environmental from a community perspective: the future is boundless and vivid. Ecosystems 19:753–seventy. doi: x.1007/s10021-016-9967-0
[iii] ↑ Brady, N., and Weil, R. 2010. "Nutrient cycles and soil fertility," in Elements of the Nature and Properties of Soils, 3rd Edn, ed V. R. Anthony (Upper Saddle River, NJ: Pearson Education Inc.), 396–420.
[iv] ↑ Foth, H. 1990. Chapter 12: "Plant-Soil Macronutrient Relations," in Fundamentals of Soil Scientific discipline, 8th Edn, ed John Wiley and Sons (New York, NY: John Wiley Company), 186–209.
[5] ↑ Chislock, Chiliad. F., Doster, Eastward., Zitomer, R. A., and Wilson, A. East. 2013. Eutrophication: causes, consequences, and controls in aquatic ecosystems. Nat. Educ. Knowl. four:10. Available online at: https://world wide web.nature.com/scitable/cognition/library/eutrophication-causes-consequences-and-controls-in-aquatic-102364466
[6] ↑ Peoples, One thousand. B., Herridge, D. F., and Ladha, J. K. 1995. Biological nitrogen fixation: an efficient source of nitrogen for sustainable agronomical product? Plant Soil 174:3–28. doi: ten.1007/BF00032239
[7] ↑ Manahan, S. East. 2010. Environmental Chemical science, 9th Edn. Boca Raton, FL: CRC Printing, 166–72.
[8] ↑ Haycock, N. East., and Pinay, K. 1993. Groundwater nitrate dynamics in grass and poplar vegetated riparian buffer strips during the winter. J. Environ. Qual. 22:273–8. doi: 10.2134/jeq1993.00472425002200020007x
Source: https://kids.frontiersin.org/articles/10.3389/frym.2019.00041
0 Response to "How Nutrients Are Moved From Available to Unavailable Soil Pools and Then Back Again."
Post a Comment